PREAMBLE
Under the auspices of the American College of Gastroenterology (ACG) Practice Parameters Committee, a group of experts was identified for the writing group of this guideline document. The proposed writing group was reviewed by the ACG Practice Parameters Committee and the ACG leadership, and the final approved writing group consisted of the current authorship team.
Regular meetings were conducted among this writing group throughout the guideline development process to formulate Population, Intervention, Comparison, and Outcome questions that guided the subsequent literature search; development of recommendation statements and key concepts; Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) assessments; and the preparation of the full guideline document. Electronic literature searches were conducted in PubMed (MEDLINE), EMBASE, and the Cochrane Library. The search strategy was developed and executed in PubMed (MEDLINE) and then adapted to the syntax and controlled vocabulary of Embase and Cochrane Library. The searches were filtered to fully published articles on human populations in the English language, with a focus on the highest levels of evidence. Priority was given to systematic reviews and meta-analyses, followed by randomized controlled trials whenever available.
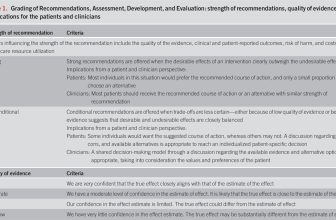
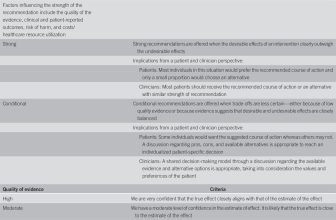
The GRADE process was used to assess the quality of evidence for each guideline recommendation (Table 1) (1–4). The quality of evidence is expressed as high (we are confident in the effect estimate to support a particular recommendation), moderate, low, or very low (we have very little confidence in the effect estimate to support a particular recommendation) based on the risk of bias of the studies, evidence of publication bias, heterogeneity among studies, directness of the evidence, and precision of the estimate of effect (2). A strength of recommendation is given as either strong (recommendations) or conditional (suggestions) based on the quality of evidence, risks vs benefits, feasibility, and costs considering perceived patient-based and population-based factors (5). Furthermore, a narrative evidence summary for each section provides important definitions and further details for the data supporting the statements.

Grading of Recommendations, Assessment, Development, and Evaluation: strength of recommendations, quality of evidence, and implications for the patients and clinicians
In addition to guideline recommendations, which are summarized in Table 2, the authors formulated key concepts that were deemed clinically important by the content authors (Table 3), but which were not amenable to GRADE assessment because of the limited available literature and/or the structure of the Population, Intervention, Comparator, and Outcome question of interest. Key concepts may include both expert opinion recommendations and definitions/epidemiological statements. Based on the guideline recommendations and key concepts, a synthesis of risk assessment and management is shown algorithmically in Figure 1.

Summary and strength of recommendations

Key concepts

Proposed algorithm for cirrhosis surgical risk assessment and management. CSPH, clinically significant portal hypertension; HE, hepatic encephalopathy; MELD, Model for End-Stage Liver Disease; PLT, platelet; SBP, spontaneous bacterial peritonitis; TIPS, transjugular intrahepatic portosystemic shunt.
INTRODUCTION
Patients with cirrhosis have increased surgical risk relative to patients without cirrhosis. Given an increasing prevalence of cirrhosis and aging cohorts of patients with cirrhosis, the absolute volume of cirrhosis surgeries has been increasing (6,7). Thus, it is critical for clinicians to understand the elements of risk stratification and perioperative risk reduction that pertain to diverse patients with cirrhosis undergoing surgery. In this guideline, we comprehensively review established factors contributing to operative risk in cirrhosis, propose essential elements of the preoperative evaluation, and address measures to mitigate operative risk. Several surgery-specific considerations are also discussed.
RISK FACTORS FOR ADVERSE POSTOPERATIVE OUTCOMES IN PATIENTS WITH CIRRHOSIS
Overview of pathophysiological basis of increased surgical risk in cirrhosis and risk estimation
Key concepts
The pathogenetic mechanisms by which cirrhosis results in adverse surgical outcomes are complicated and multifactorial. Portal hypertension, a key hallmark of advanced cirrhosis, contributes to risk through multiple pathways. The presence of portosystemic collaterals increases the risk of intraoperative and postoperative bleeding, whereas ascites can lead to infection, impaired wound healing, and leakage from surgical incisions—particularly in abdominal procedures. Cirrhosis is a major predisposing condition for malnutrition, frailty, and sarcopenia and can directly manifest with protein synthetic dysfunction. These conditions impair wound healing and physical recovery after surgery. Hematological abnormalities caused by cirrhosis further compound operative risk. Thrombocytopenia interferes with primary hemostasis, and disruptions in secondary hemostasis can result in either bleeding or thrombosis, reflecting the delicate balance of coagulopathy in cirrhosis. In addition, cirrhosis is associated with renal and metabolic derangements, including electrolyte abnormalities and a predisposition to acute kidney injury and hepatorenal syndrome, all of which may complicate the perioperative course. The immunological dysfunction observed in cirrhosis increases the susceptibility to wound infections and sepsis, and cardiovascular dysfunction, including hypotension and cirrhotic cardiomyopathy, may contribute to intraoperative hemodynamic compromise and/or postoperative heart failure. Finally, neurological complications such as hepatic encephalopathy may be precipitated or worsened by surgery, complicating recovery and increasing the risk of poor outcomes.
Although the pathogenetic mechanisms are complex, in practice, only a small number of “predictors” are readily available for postoperative risk prediction (8–12). A key concept is that risk factors known to be associated with adverse postoperative outcomes in cirrhosis fall into 3 distinct categories, as shown in Figure 2: (i) liver-related factors, (ii) nonhepatic comorbid conditions and patient factors, and (iii) surgery-specific factors.

Domains and risk factors associated with operative risk in patients with cirrhosis. ASA, American Society of Anesthesiologists.
The only way to make accurate individualized predictions is by using cirrhosis-specific surgical risk calculators that incorporate the critical predictors across all 3 categories to yield a composite score. General scores of liver function or liver disease severity (such as the Model for End-Stage Liver Disease [MELD]-Na or Childs-Turcotte-Pugh [CTP] score) are inadequate because they do not incorporate the complexity of the surgical procedure (e.g., a person undergoing a very complex surgery such as Whipple surgery will have the same “score” as a person undergoing a relatively simple surgery such as hernia repair) or risk related to extrahepatic comorbidities (e.g., a person with multiple non–liver-related comorbidities will have the same score as a person with no other comorbidities). Moreover, generic (i.e., non–cirrhosis-specific) surgical risk calculators such as the American College of Surgeons National Surgical Quality Improvement Program calculator (13) are inadequate because they do not include detailed measures of liver dysfunction that are critical in evaluating surgical risk in patients with cirrhosis. Cirrhosis-specific models that address liver-related factors, nonhepatic comorbid conditions, and surgery-specific factors have been recently developed and validated, such as the VOCAL-Penn Score (10), which uses 8 readily available factors combined into models estimating postoperative mortality and decompensation.
Synthesis of established risk factors affecting surgical risk in cirrhosis
Key concepts
Some studies suggest that patients with cirrhosis who have very low MELD scores (6–9) and no evidence of cirrhosis decompensation (i.e., no ascites, encephalopathy, or variceal bleeding) or clinical evidence of portal hypertension without decompensation (i.e., varices, portosystemic collaterals) have little excess surgical risk, especially for low-risk, elective surgeries (14,15). For example, in patients with cirrhosis without ascites who underwent elective umbilical hernia repair, the 30-day mortality was low at 0.7% (although still higher than that in matched noncirrhosis patients who had 0% mortality) (9). In patients with cirrhosis and MELD 6–9 who underwent any abdominal surgery, the 30-day mortality was also low at 1.2%, but still higher than that in noncirrhosis controls (0.8%) (11).
Cirrhosis is increasingly being diagnosed at very early stages using noninvasive methods such as elastography. Recent guidelines emphasized the concepts of compensated advanced chronic liver disease (cACLD) and CSPH and proposed noninvasive diagnostic criteria based on liver stiffness and platelet (PLT) counts (16). These criteria allow identification of patients with advanced liver disease or cirrhosis who have cACLD but who do not have CSPH or any evidence of decompensated cirrhosis. This includes patients with cirrhosis who have transient elastography liver stiffness measurements (LSMs) 10–14.9 kPa, LSM 15–19.9 kPa with PLTs >110 K/mm3, or LSM 20–24.9 kPa with PLTs >150 K/mm3. It is possible that such patients with cACLD but without evidence of CSPH or decompensation and near normal MELD (6–9) and CTP scores (5–6) have limited excess risk of adverse surgical events. However, this remains to be proven as these concepts are relatively novel.
Key concepts
The hepatic venous pressure gradient (HVPG) is the gold-standard method to assess portal pressure in cirrhosis. Portal hypertension is defined by HVPG ≥5 mm Hg, whereas HVPG ≥10 mm Hg is used to define CSPH. The presence of gastroesophageal varices, portosystemic collaterals, or hepatofugal flow in the portal vein is sufficient to diagnose CSPH. Patients with clinical evidence of hepatic decompensation (ascites, encephalopathy, bleeding varices) by definition have CSPH that has also manifested in adverse clinical presentations. HVPG is measured by hepatic vein catheterization as the difference between the wedged and free hepatic venous pressure; hence, it is performed infrequently in most practices. A major recent advancement is the proposal of noninvasive definitions of CSPH based on transient elastography LSM and PLT count (LSM ≥25 kPa irrespective of PLT count, LSM 20–24.9 kPa with PLT count <150 K/mm3, or LSM 15–19.9 kPa with PLT count <110 K/mm3) (16,17).
Portal hypertension is one of the most important risk factors for adverse postoperative outcomes, with higher levels of portal hypertension conferring higher levels of risk (12,18). This is particularly true of abdominal surgeries, for which the presence of ascites and portosystemic collaterals pose specific risks above and beyond those for nonabdominal surgeries (11), including an increased risk of bleeding and more challenging surgical hemostasis. Although not part of routine care in the United States, HVPG is an independent predictor of outcomes after surgery in patients with cirrhosis (19). HVPG was found to predict postoperative outcomes after hepatic resection, independent of thrombocytopenia, splenomegaly, or varices (20). HVPG is also associated with increased risk of liver-related adverse events postoperatively in patients with cirrhosis undergoing extrahepatic surgery (5). The presence of CSPH confers an increased surgical risk even without concomitant decompensating events (e.g., varices without bleeding or HVPG ≥10 mm Hg without decompensating events), albeit at a lower level. It is unknown whether the new definitions of CSPH based on combinations of LSM and PLT count thresholds also predict perioperative risk, particularly with limitations of operator dependence for LSM. However, thrombocytopenia has been shown to independently predict adverse postoperative outcomes (10,11,18).
Key concepts
There is little evidence that etiology of liver disease in patients with cirrhosis independently affects surgical risk. One exception may be metabolic dysfunction–associated steatotic liver disease (MASLD)–related cirrhosis, which is associated with multiple adverse cardiometabolic factors (diabetes, insulin resistance, metabolic syndrome, central/visceral obesity) that may independently affect surgical outcomes negatively but are challenging to account for comprehensively. Previous studies that simultaneously adjusted for multiple cardiometabolic outcomes did not find a significant association between liver disease etiology and postoperative mortality (11). However, the VOCAL-Penn cirrhosis surgical risk score does include a “MASLD” variable and assigns a slightly higher risk score for patients with MASLD cirrhosis (21); this variable likely serves as a surrogate for cardiometabolic risk factors.
It is now well recognized that changes in both anticoagulant/profibrinolytic and procoagulant/antifibrinolytic pathways occur in cirrhosis, resulting in complex negative and positive effects on hemostasis, which are not accurately captured by traditional laboratory tests such as PT/INR, aPTT, and PLT count (22). Studies of the associations between PLT count and procedural bleeding in cirrhosis reported mixed results (22–26) but overall tend to suggest that very low PLT counts (<50/μL or <75/μL) are indeed associated with higher risk of bleeding (25). However, a low PLT count may also reflect increased severity of liver disease and portal hypertension, which would not “correct” with interventions aimed at increasing the PLT count. Furthermore, the PLT count by itself does not precisely mirror PLT function, for example, it does not account for elevations in the PLT adhesive protein von Willebrand factor which increases in cirrhosis and tends to compensate for thrombocytopenia from a standpoint of bleeding risk.
Coagulopathy as measured by elevated levels of PT/INR or PTT has not been independently associated with periprocedural bleeding in cirrhosis. This is not surprising because PT and PTT are only sensitive to procoagulant proteins (decreased in cirrhosis) but not anticoagulant factors (also decreased in cirrhosis). Furthermore, INR is an important component of both the MELD and CTP scores because it is a very strong marker of liver dysfunction, which would not improve by measures aimed at directly correcting the INR.
Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are point-of-care, whole-blood viscoelastic tests that provide a global functional assessment of clot formation and dissolution. These measures may in theory be superior tools for hemostatic balance assessment in cirrhosis than PT/aPTT or PLT count. However, TEG and ROTEM are insensitive to von Willebrand factor and protein C and therefore may underestimate hemostatic function in patients with cirrhosis.
Key concepts
Nonhepatic comorbid conditions affect postoperative mortality and morbidity in patients with cirrhosis, just as they do in patients without cirrhosis. Overall measures of functional status and comorbidity such as American Society of Anesthesiologists (ASA) class (10,11), Charlson comorbidity index (27), and older age (9,10), as well as individual chronic conditions such as chronic obstructive pulmonary disease, heart failure, and cancer, and acute conditions such as sepsis and ventilator dependence (11), greatly affect postoperative mortality.
Key concepts
Cirrhosis predisposes to malnutrition, sarcopenia, and frailty, which are now increasingly recognized as potentially modifiable causes of adverse outcomes in cirrhosis (28). The prevalence of malnutrition (∼80%) (29) and sarcopenia (25%–70%) (30) is very high in cirrhosis. Sarcopenia (which may be assessed by imaging, bioimpedance, or anthropometry) can occur even in normal or elevated body mass index and is associated with delayed wound healing and poor surgical outcomes (31,32). Frailty, defined as a clinical state of decreased physiological reserve and increased vulnerability to health stressors, has also been associated with adverse postoperative outcomes in cirrhosis (33,34). Frailty may be assessed by the presence of relevant diagnostic codes combined into a risk score (e.g., VA Frailty index and Hospital Frailty Risk Score) (34–38) or through performance of dedicated frailty tests such as the Liver Frailty Index (grip strength, chair stands, and balance testing) (39) which has been shown to be associated with postoperative mortality after liver transplant (40).
PREOPERATIVE EVALUATION
Noninvasive fibrosis assessment to identify advanced fibrosis/cirrhosis
Key concepts
Among patients with known or suspected chronic liver disease, without a diagnosis of cirrhosis, assessment of hepatic fibrosis may inform the decision to proceed with surgery or alter the nature of the planned operation, by identifying patients with previously undiagnosed cirrhosis. Fibrosis staging can be performed using widely available noninvasive tests, including biomarker panels (e.g., FIB-4) or elastography (e.g., vibration-controlled transient elastography, ultrasound, or MRI). These tests have adequate negative predictive value for the reasonable exclusion of advanced fibrosis/cirrhosis and have been shown to be associated with postoperative outcomes (19,41–44). If surgical plans or patient counseling would not change with a new diagnosis of compensated cirrhosis, there is no need for preoperative assessment for hepatic fibrosis.
Applying prognostic models for cirrhosis surgical risk
Key concepts
As noted previously, assessment of surgical risk among patients with cirrhosis requires understanding of the severity of the patient’s underlying liver disease, nonhepatic comorbid factors, and unique risks of the planned surgery. It is well established that perioperative risk increases with severity of liver disease. This phenomenon can be as assessed by familiar clinical indices such as CTP (20), MELD (45,46), Albumin-Bilirubin (47), and Chronic Liver Failure-Consortium (48), among others. Fundamentally, these models use readily available laboratory and clinical data to assess functional capacity of the liver (e.g., INR, albumin) and severity of portal hypertension (e.g., PLT count). There are 2 models which have been developed specifically for perioperative risk assessment in patients with cirrhosis to provide discrete predictions of postoperative mortality: the Mayo Surgical Risk Score (46) and the VOCAL-Penn Score (10). While the Mayo Risk Score accounts for both severity of liver disease and nonhepatic comorbidities (via age and ASA classification variables), this score has become progressively miscalibrated over time and has been demonstrated in multiple studies to overestimate surgical risk (10,49,50). The VOCAL-Penn Score is the most recently developed and adopted cirrhosis surgical risk calculator and is unique in its incorporation of surgery type into risk predictions. This is critical as perioperative risks may vary widely based on the planned surgery (51). There has been direct comparison of the performance of these models, which suggests that the predictive ability of the VOCAL-Penn Score exceeds that of the Mayo Surgical Risk Score in discrimination, calibration, and overall performance (10,50,52). An additional unique benefit of the VOCAL-Penn Score is prediction of postoperative decompensation through 90 days (18) and its applicability to both nonhepatic and liver-specific surgeries (53). Both prediction models are freely available as online calculators (21,54). Importantly, given that cirrhosis surgical risk scores were derived and validated only in patients who actually underwent surgery, these scores should never be used in isolation from clinical judgment. Rather, they should be used as an adjunct tool to assist the surgery and hepatology teams in comprehensive preoperative evaluation and risk stratification and in shared decision-making conversations with patients. Variables incorporated into commonly used cirrhosis surgical risk scores are summarized in Table 4.

Variables comprising commonly used cirrhosis surgical risk scores
Preoperative assessment for CSPH
Recommendation
CSPH has important prognostic implications for liver-related adverse postoperative events (12,55,56). Although there is a paucity of data regarding the impact of preoperative CSPH assessment on perioperative risk for extrahepatic surgeries, several studies have addressed this question for liver resection (57–59). Recently proposed noninvasive criteria for the presence of CSPH (see Synthesis of established risk factors above) are useful in predicting postoperative outcomes in patients undergoing hepatectomy, with particularly strong predictive value at more extreme values (57). However, patients with LSM between approximately 14 and 25 kPa without decompensation may benefit from direct measurement of HVPG if available locally and, importantly, if confirmation of the absence of CSPH would lead to change in management.
Liver transplant evaluation before elective surgery
Key concepts
There are no standardized criteria to prompt liver transplant evaluation before surgery in patients with cirrhosis although previous expert guidance has recommended considering evaluation in patients with a projected 90-day mortality over 15% (60). It is implicit that transplant listing only occurs in patients without obvious contraindications, for example, prohibitive and unmodifiable cardiovascular or psychosocial comorbidities. Patients in need of emergency surgery might not have time to complete transplant evaluation preoperatively; however, it may still be relevant to discuss potential transplant candidacy if there is a concern for major risk of postoperative decompensation. Such patients may benefit from consultation with or transfer to a center that offers liver transplant to permit expedited evaluation in the postoperative setting, if needed. Even in the emergent setting, it may be feasible to complete an abbreviated evaluation consisting of key components, for example, cardiac risk assessment. Local expertise should govern identification of an appropriate compromise between the financial and logistical costs of a full transplant evaluation and the benefits of identifying major barriers to transplant candidacy preoperatively.
For patients undergoing elective surgery at high risk of hepatic decompensation but who do not otherwise meet criteria for liver transplantation, completing a transplant evaluation preoperatively is advisable to avoid extensive testing in the midst of a postoperative decline. Such patients need not be immediately waitlisted for transplant; however, knowledge of suitability for transplant may inform decision-making by both patients and providers in advance of high-risk surgery. Finally, in specific scenarios where surgical pathology results may ultimately determine transplant eligibility—for example, patients with cirrhosis and colon cancer who require colectomy to establish cancer staging—it is essential to engage in detailed preoperative discussions regarding prognosis and transplant candidacy. This includes counseling on the possibility that if surgical findings render the patient ineligible for transplant (e.g., cancer staging beyond transplant criteria), decompensation after surgery might have significant implications for long-term outcomes and quality of life.
Decisions about whether to recommend surgery to a patient with cirrhosis must balance the expected risks of the surgery (generally and those unique to patients with cirrhosis) against the risk of the underlying condition for which surgery is recommended. Accurate estimation of perioperative risk using models previously discussed may help avoid excess concern, which may limit access to otherwise important care for patients with cirrhosis.
Assessment of preoperative frailty
Key concepts
Assessment of frailty using the Hospital Frailty Risk Score was found to improve prediction of postoperative mortality in patients with cirrhosis undergoing extrahepatic surgery as compared with MELD alone (34); however, this did not improve predictive capacity beyond that of models designed for cirrhosis surgical risk prediction including MRS and the VOCAL-Penn Score (34). It is unclear if in-person assessments of frailty using cirrhosis-specific instruments such as the liver frailty index may improve risk stratification in this setting.
RISK MITIGATION STRATEGIES AND PERIOPERATIVE MANAGEMENT
In the context of careful preoperative risk assessment, it is important to consider modifiable risk factors and pursue interventions that reduce the risk of postoperative mortality and morbidity. Interventions to be considered may include treatment of reversible aspects of underlying liver disease, lifestyle changes, correction of clinically relevant hemostatic derangements, medical optimization of cirrhosis-related decompensation events, and potential measures to reduce portal hypertension.
Treatment of underlying liver disease
Key concepts
When possible, patients with decompensated cirrhosis or who have reversible hepatic insults, for example, active alcohol use, hepatitis B, hepatitis C, or autoimmune hepatitis, should receive treatment for these conditions before elective surgery in an effort to improve liver function. Similarly, any opportunities to minimize perioperative risk should be discussed in multidisciplinary consultation between Surgery, Anesthesiology, and Hepatology teams and potentially others, for example, Interventional Radiology, as indicated. If no options to improve liver function preoperatively exist or if such efforts would result in unacceptable delay, risk can be assessed using the aforementioned predictive models and a decision can be made based on patient and provider interpretation of risk and benefit balance. Such decisions should also incorporate assessment of the risks associated with avoidance of the intended surgery itself.
Alcohol and tobacco cessation
Key concepts
The adverse impacts of significant preoperative alcohol use are well established in general surgical populations (61–63). Alcohol use in patients with cirrhosis has deleterious effects directly on liver function and portal hypertension in addition to adverse systemic effects. In a large meta-analysis of broadly selected patients, active preoperative alcohol consumption was associated with increased risk of infection (relative risk [RR] 1.73, 95% confidence interval [CI] 1.32–2.28), wound complications (RR 1.23, 95% CI 1.09–1.40), ICU admission (RR 1.29, 95% CI 1.03–1.61), and prolonged length of stay (RR 1.24, 95% CI 1.18–1.31) (64). In patients with liver disease and hepatocellular carcinoma undergoing liver resection, preoperative tobacco use was an independent risk factor for liver-related complications (65) and concomitant tobacco and preoperative alcohol use in general populations demonstrates synergistic increases in postoperative risk (66). It is therefore reasonable to recommend alcohol and tobacco cessation in patients with cirrhosis being considered for elective surgery with period of recommended cessation determined by the urgency of the surgery balanced against accrual of benefit of sobriety over time.
Nutritional optimization
Key concepts
Limited data suggest that perioperative nutritional support initiated 14 days before surgery may reduce major postoperative morbidity rates in patients with cirrhosis (RR 0.66, 95% CI 0.45–0.96) (67). Patients should consume at least 30–35 kcal/kg calories per day and 1.25–1.5 g/kg per day of protein, calculated based on ideal body weight. Protein intake should not be restricted in patients with hepatic encephalopathy, and a late evening snack may improve total body protein status by shortening periods of nocturnal fasting (68). Preoperative nutrition consultation and placement of a nasoenteric tube may be needed in some patients to meet the above goals; the latter is not contraindicated by the presence of nonbleeding esophageal varices (28). Finally, although there are very limited data outside the context of liver transplant in patients with cirrhosis (69), prehabilitation programs designed to improve preoperative exercise capacity and functional status have been demonstrated to decrease incidence of postoperative complications and reduce healthcare utilization in heterogeneous cohorts (70–72) including those waitlisted for liver transplantation (73–75).
Management of hemostatic derangements
Key concepts
Derangements in hemostatic parameters are common in patients with cirrhosis; however, as noted the data evaluating bleeding risk as a function of preoperative PLT count in cirrhosis are mixed. Several prospective studies of patients with cirrhosis found no difference in periprocedural bleeding related to PLT count, including in those with PLT <50 K/mm3 (23,24). However, in another study of patients with cirrhosis undergoing invasive procedures, a PLT count of <75 K/mm3 was independently associated with periprocedural bleeding, but significant coagulopathy (INR >1.5) was not (25). A challenge in interpreting the literature is heterogeneity in the use of prophylactic PLT transfusions, which may paradoxically increase bleeding risk related to exacerbation of portal hypertension (22,26). Finally, Vitamin K administration has not been studied in the preoperative setting; however, routine use does not reduce the risk of bleeding in hospitalized patients with cirrhosis (76,77).
Viscoelastic testing such as TEG or ROTEM and point-of-care tests that provide a global functional assessment of clot formation and dissolution may be a superior tool to guide transfusion as compared with traditional hemostatic parameters. Several randomized controlled trials (RCTs) and a meta-analysis have demonstrated reduced utilization of blood product transfusions without increased incidence of bleeding when TEG was evaluated in patients with cirrhosis undergoing invasive procedures (78–80). In an RCT of 58 patients with cirrhosis, only 31% of patients received preprocedural blood product transfusions when guided by TEG parameters vs 100% in the standard-of-care group, and no patients in either group developed periprocedural bleeding (80). Although TEG has not been studied in patients with cirrhosis undergoing major surgery, it is plausible that a TEG-guided transfusion approach would also reduce unnecessary transfusions in this setting. While there is limited evidence or consensus-based guidance to inform specific considerations as to the optimal timing of viscoelastic testing, in practice, timing may depend on local protocols, surgical timing, and the clinical status of the patient.
Use of thrombopoietin receptor agonists to mitigate preoperative thrombocytopenia
Recommendation
Use of TPO receptor agonists such as avatrombopag and eltrombopag are effective in transiently raising the PLT count and have been studied in patients with cirrhosis and thrombocytopenia undergoing elective invasive procedures. In an RCT of 292 patients with cirrhosis and PLT count <50 K/mm3, those randomized to eltrombopag (75 mg daily for 14 days) in advance of invasive procedures were less likely to receive PLT transfusion than those randomized to placebo (28% vs 81%, P < 0.001). There were no significant differences in bleeding episodes; however, portal venous thrombotic events were observed in 5.7% of eltrombopag recipients vs 0.7% placebo (81). In a similar study, a 5-day course of avatrombopag initiated at 60 mg daily for baseline PLT <40 K/mm3 or 40 mg daily for baseline PLT 40–50 K/mm3 demonstrated a significant reduction in PLT transfusion vs placebo (e.g., 11.9% 40 mg dose vs 61.8% placebo, P < 0.001) and no increased risk of thrombotic adverse events (82). Of note, in the eltrombopag study, portal venous thromboses were attributed to more aggressive dosing with PLT counts rising to >200 K/mm3 in some patients. By contrast, dosing of avatrombopag in the latter study yielded average PLT peaks of ∼65–90 K/mm3 depending on dosing and baseline PLT counts. In a recent meta-analysis of 6 studies, use of TPO receptor agonists in patients with cirrhosis and PLT <50 K/mm3 was significantly associated with increased preoperative PLT count, reduced incidence of PLT transfusions (22.5% vs 67.8%, P < 0.001), and reduced incidence of periprocedural bleeding (11.6% vs 15.6%, P = 0.01) (83).
Role of preoperative TIPS in addressing portal hypertension
Recommendation
Patients with cirrhosis undergoing major abdominal or thoracic cavity surgeries are particularly susceptible to portal hypertension-related risks of bleeding and difficult intraoperative hemostasis. Preoperative TIPS is therefore a candidate intervention to mitigate this risk in this setting. Data on this practice are mixed, and there are no prospective studies or trials to inform a robust evidence base. Early studies of patients with cirrhosis undergoing major surgery demonstrated no significant difference in postoperative mortality with preoperative TIPS placement (84,85), including in matched or adjusted analyses (86,87). However, more recently, in a propensity-matched analysis, Chang et al (48) reported lower rates of postoperative acute-on-chronic liver failure in patients with cirrhosis undergoing diverse surgeries who received preoperative TIPS (9% vs 29% at 28 days, P = 0.02), and in a multivariable adjusted analysis, Piecha et al (88) demonstrated lower in-hospital mortality and need for liver transplant after both low- and high-risk surgeries (primarily chest and abdominal) in patients with preoperative TIPS (19% vs 40%, P = 0.003). Of note, 41% of TIPS placed in the preoperative period was placed primarily for preoperative risk reduction, 38% was placed for refractory ascites, and 9% was placed after variceal hemorrhage. While these results are encouraging, both these studies were single-center studies and concerns remain regarding potential residual confounding and possible inappropriate adjustment of mediators (e.g., immediate preoperative laboratory tests). Indeed, in a recent study where preoperative TIPS patients were propensity matched to controls based on data obtained 6 months before surgery, TIPS patients had evidence of worsened liver synthetic function immediately before surgery and increased hazard of postoperative mortality (hazard ratio 2.69, 95% CI 1.37–5.30, P = 0.004) (89). This suggests that operative benefits of TIPS-induced portal pressure reduction may be offset to some degree by changes in hepatic perfusion. Thus, in the absence of higher-quality and ideally prospective data, utilization of preoperative TIPS should be limited to experienced centers and decided on a patient-by-patient basis through multidisciplinary discussion. Alternative indications for TIPS (e.g., large varices and previous variceal bleeding or refractory ascites) and contraindications (e.g., refractory hepatic encephalopathy) should also be considered in this decision-making process. Patients with refractory ascites undergoing consideration of surgery may benefit from preoperative TIPS from both ascites and perioperative risk standpoints, whereas patients with established contraindications such as heart failure, severe tricuspid regurgitation, or polycystic liver disease should not undergo TIPS.
Performance of surgery at an experienced center
Recommendation
Key concepts
Given the unique operative risks in patients with cirrhosis, it is plausible that surgery performed at high-volume and/or transplant centers may lead to improved outcomes. Data on this subject are largely focused on hepatic surgery. For instance, in a National Cancer Database analysis of 12,757 patients who underwent liver resection for hepatocellular carcinoma, high-volume centers (defined as ≥10 hepatectomies per year) had a higher adjusted 10-year survival rate vs low-volume centers (28.7% vs 25.1%, P < 0.001) (90). In a French multicenter study of 39,286 liver resection patients (37% with chronic liver disease including cirrhosis), high-volume centers (defined as ≥25 hepatectomies per year) had a significantly lower odds of in-hospital mortality relative to lower-volume centers (odds ratio 0.79, 95% CI 0.65–0.97, P < 0.001) (91). Similar findings have been reported in analyses of the National Inpatient Sample (92,93) and in a multicenter Italian cohort of 1,935 patients who underwent liver resection for HCC (94). This study reported lower adjusted mortality rates and lower incidence of failure to rescue at high-volume centers. Regarding nonhepatic surgeries, an National Inpatient Sample analysis of patients with cirrhosis undergoing orthopedic procedures demonstrated an inverse relationship between hospital cirrhosis volume and in-hospital mortality (95). The data thus suggest that it is reasonable to refer patients with cirrhosis planned for major surgery to an experienced center when feasible.
Preoperative management of cirrhosis-related decompensations
Key concepts
While there are limited data evaluating in detail the perioperative management of cirrhosis decompensations, there are several principles that should be followed. Ascites, when present, should be preoperatively sampled to rule out SBP. Active SBP should prompt delay of elective surgery until a course of appropriate antibiotics has been completed. In patients without active SBP, indications should be reviewed for initiation of SBP prophylaxis, including previous SBP and low-protein ascites (<1.5 g/L) with concomitant renal dysfunction (creatinine >1.2 mg/dL, blood urea nitrogen >25 mg/dL, or serum sodium <130 mEq/L) or liver failure (CTP score >9 and bilirubin >3 mg/dL) (96). Large-volume ascites should be preoperatively drained (albumin 6–8 g/L should be given if >5 L is removed) and actively managed in the postoperative setting. This is especially critical for abdominal surgeries, where recurrent tense ascites increases the risk of wound dehiscence and surgical site infection. In the absence of preoperative acute kidney injury or major electrolyte derangements, diuretics (if prescribed) should be continued in the perioperative period. Patients with hepatic encephalopathy should have lactulose and/or rifaximin continued through the perioperative period, with close attention to serum sodium and minimization of medications that may exacerbate encephalopathy. Patients taking nonselective beta blockers (NSBBs, e.g., carvedilol) for CSPH or secondary prevention of esophageal variceal bleeding should continue these medications in the peri-operative period, provided that the systolic blood pressure is over 90 mm Hg. In patients not taking NSBBs and with unclear variceal status, upper endoscopy should be performed and NSBBs or band ligation should be pursued for large varices or medium varices with high-risk stigmata. Finally, hepatocellular carcinoma screening should be up-to-date, especially as diagnosis of liver cancer may affect surgical decision-making and transplant consideration in selected patients. The importance of the above routine management is underscored by data demonstrating that patients with recent outpatient gastroenterology/hepatology visits (along with active management of cirrhosis-related medications and paracentesis) before surgery have lower postoperative mortality relative to patients without recent outpatient visits (97).
SURGERY-SPECIFIC CONSIDERATIONS
In addition to the above general guidance on preoperative risk evaluation and risk mitigation strategies, there are several specific surgical scenarios that are uniquely affected by cirrhosis, which warrants additional discussion.
Abdominal hernia repair
Key concepts
The prevalence of abdominal hernia can reach up to 40% in patients with decompensated cirrhosis (98). Although there are no strict contraindications for elective repair, management is often deferred because of the presence of severe hemostatic derangements, CSPH, refractory ascites, and/or malnutrition/sarcopenia. Conservative management additionally avoids the risks of anesthesia, which is an important consideration given the degree of systemic disease and high ASA score in many patients with decompensated cirrhosis. However, deferring operative repair also increases the risk of complications requiring emergent repair including incarceration, strangulation, spontaneous or threatened rupture, evisceration, gangrenous skin changes, or secondary peritonitis.
The decision to pursue elective abdominal hernia repair in cirrhosis is a common clinical dilemma. In the CRUCIAL randomized controlled trial of 34 adults with liver cirrhosis and umbilical hernia, there was no significant difference in time to minor or major complications between elective repair and conservative management through a 2-year follow-up (overall complication rate 64.7%; group difference P = 0.66) although the study might have been underpowered. Among those who underwent repair, recurrent hernia occurred in 16.7% (99). A 2022 systematic review of 23 studies comprising 3,229 patients undergoing hernia repair demonstrated an increased risk of mortality in patients with cirrhosis (odds ratio 8.50, 95% CI 1.91–37.86); however, much of the increased risk was attributed to emergent hernia repair (100). This point is underscored by a Veterans Health Administration study of 1,475 patients with cirrhosis who underwent open umbilical hernia repair. Patients who underwent emergency hernia repair had significantly higher 30-day mortality than those who underwent elective repair (12.2% vs 1.2%), and of patients who underwent elective repair, mortality was higher in patients with ascites (2.2% vs 0.7%). Most importantly, chart review revealed that elective umbilical hernia repair might have been feasible in 30% of patients requiring emergency umbilical hernia repair in the previous year (9). Another Veterans Health Administration study similarly demonstrated that elective hernia repair would yield higher quality-adjusted life years in patients with cirrhosis and a MELD score of up to 21 in a Markov decision analysis. The literature therefore suggests that elective hernia repair should be considered in patients with cirrhosis with close monitoring as liver disease progresses. For patients on the transplant waiting list who are expected to undergo transplant within 6 months, hernia repair can be deferred to the transplant operation. Findings such as thinning skin over the hernia or leakage of ascites warrant close observation and consideration of emergent repair to avoid rupture (101).
Ascites control is also of particular importance in patients with cirrhosis undergoing elective hernia repair as inadequate control increases risk of wound complications and hernia recurrence that may drive morbidity and mortality. Despite the importance of adequate preoperative ascites control, there is a paradoxical risk of hernia incarceration with rapid ascites reduction because of decompression and reduction of the abdominal wall defect. The approach to ascites control should follow standard management algorithms for patients with decompensated cirrhosis (102), which may include sodium restriction, loop and antimineralocorticoid diuretics, and large volume paracentesis as indicated. As noted above, TIPS may also be considered to reduce portal pressures on a case-by-case basis in appropriate patients. For patients with persistent ascites despite these measures, perioperative drainage can be used to temporarily divert ascites away from the operative site. There is insufficient evidence to support use of drains in the postoperative period after umbilical hernia repair; however, patients with perioperative drains should be closely monitored for hypovolemia and electrolyte imbalance because of fluid shifts (102).
Cholecystectomy
Key concepts
The prevalence of cholelithiasis is approximately 2-fold higher in patients with cirrhosis (103); however, consistent across all populations is the consensus that patients with asymptomatic cholelithiasis should not be treated. In patients with CTP A and B cirrhosis, indications for elective cholecystectomy are the same as in patients without cirrhosis (e.g., symptomatic cholelithiasis, high-risk gallbladder polyps, etc.) and laparoscopic cholecystectomy remains the procedure of choice (104,105). Although medical therapies exist for symptomatic cholelithiasis, they have not been shown to be effective in patients with cirrhosis and reduced gallbladder motility (106).
Antibiotics and cholecystectomy are the standard of care for acute cholecystitis. As noted, laparoscopic cholecystectomy is generally safe in CTP A and B cirrhosis, but is associated with significantly higher rates of postoperative complications and mortality in CTP class C disease (107). In cases of high concern or uncertainty, estimation of postoperative mortality using the VOCAL-Penn Score may help to inform decision-making given that patients undergoing laparoscopic and open abdominal cholecystectomy were included in the derivation of this model (10). Patients with prohibitive surgical risk should be initially treated with antibiotics, and in the case of failed medical management, percutaneous cholecystostomy, transpapillary drainage, and endoscopic ultrasound-guided transmural gallbladder drainage are alternative procedural options (108). Percutaneous gallbladder aspiration has also shown to be effective (109). For patients with cirrhosis with cholecystitis and common bile duct stones, endoscopic sphincterotomy or balloon extraction is preferable to laparoscopic or open common bile duct exploration. However, risks of postsphincterotomy bleeding and pancreatitis are elevated in patients with cirrhosis and more so in patients with decompensated disease (110). Patients with a MELD score of ≥15 are additionally nearly 3 times more likely to develop acute decompensation after endoscopic retrograde cholangiopancreatography relative to those with lower MELD scores (111) although it should be noted in the general population that endoscopic retrograde cholangiopancreatography may reduce risks of hospital readmission in patients with choledocholithiasis-related complications who do not undergo cholecystectomy (112). Thus, decision-making must incorporate RRs and benefits associated with different therapeutic approaches. Finally, although data are limited, cholecystectomy after endoscopic sphincterotomy has been demonstrated to reduce major complications such as recurrent choledocholithiasis, ascending cholangitis, and gallstone pancreatitis as compared with endoscopic sphincterotomy alone, including in patients with advanced liver disease (113). Thus, this option should be considered in patients with acceptable surgical risk after recovery from the index biliary event.
Cardiac surgery
There are very little data related to cardiac surgery in patients with cirrhosis although operative risk in this setting increases with severity of liver disease. A recent systematic review of 13 studies evaluated postoperative complications and long-term outcomes in patients with cirrhosis who underwent cardiac surgery (114). Postoperative mortality rates were 0%–11%, 18%–50%, and 67%–100% in CTP A, B, and C patients, respectively. However, high degrees of study heterogeneity prevented analysis of specific surgical indications or the role of various anesthetic or surgical techniques in mitigating adverse outcomes. In the CASTER study, a retrospective observational cohort study of 144 adults with CTP A or B cirrhosis who underwent cardiac surgery, procedures included coronary artery bypass grafting (36%), valvular surgery (50%), and aortic procedures (14%). Both perioperative complications and in-hospital mortality were higher in CTP B vs A cirrhosis (20% vs 13%).
The European system for cardiac perioperative risk evaluation (EuroSCORE) predicts mortality after cardiac surgery; however, liver function is not included in the score (115). A retrospective cohort study of 218 adults with liver cirrhosis who underwent cardiac surgery investigated the predictive power of EuroSCORE II. Perioperative mortality rates were 8.9% in patients with Child A, 52.9% in patients with Child B, and 100% in patients Child C. EuroSCORE II showed a poor predictive value for patients with Child B and C cirrhosis (116). Ascites is also a poor prognostic sign, consistent with other surgeries. A retrospective cohort study of 69 patients with liver cirrhosis who underwent cardiac surgery between 2009 and 2018 examined the influence of preoperative ascites on postoperative complications and 30-day mortality. Transfusion requirement (78.6% vs 40.0%), acute kidney injury (78.6% vs 45.5%), ICU stay greater than 72 hours (57.1% vs 23.6%), and need for tracheostomy (28.6% vs 3.6%) were all higher in patients with ascites, who also experienced longer ICU stay (8 vs 3 days). The 30-day mortality rate was significantly higher in the ascites group than in the nonascites group (35.7% vs 5.5%, P = 0.002) (117).
Bariatric surgery
Key concepts
Bariatric surgery is considered a safe and effective treatment for obesity in selected patients with cirrhosis. A recent meta-analysis of 15 studies demonstrated a median excess weight loss of 60.44% (95% CI 44.34%–76.55%) in adults with compensated cirrhosis and remission of metabolic comorbidities in more than half of patients, including MASLD (57.9%, 95% CI 27.5%–88.3%), type 2 diabetes (58.4%, 95% CI 48.4%–68.4%), hypertension (53.1%, 95% CI 43%–63.3%), and dyslipidemia (59.8%, 95% CI 41.1%–78.5%). Importantly, however, mortality rates were significantly higher in patients with decompensated vs compensated cirrhosis (18.2% vs 0.9%) (118).
Bariatric procedures that can be performed laparoscopically include the Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, and gastric banding, each with their own distinct advantages and disadvantages. Laparoscopic sleeve gastrectomy is increasingly recognized as the bariatric modality of choice in patients with cirrhosis, with multiple cohort studies demonstrating its safety even in patients awaiting liver transplant (119–121). Relative to gastric bypass, sleeve gastrectomy has a shorter operating time and allows access to the remnant stomach and biliary system. Sleeve gastrectomy reduces the risk of malabsorption or placement of a foreign body but may predispose to bleeding risk in the setting of gastric varices. RYGB provides the greatest overall and long-term weight loss but might have a greater risk of vitamin deficiencies that may exacerbate hepatic dysfunction. RYGB also prevents native endoscopic access to the stomach or biliary tree in cases of a gastrointestinal bleed or biliary complications. Gastric banding is the least invasive procedure, but has the lowest efficacy related to long-term weight loss and adds a potential risk of infection with placement of a foreign device (122,123); for this reason, gastric banding is infrequently performed in modern practice.
In potential liver transplantation candidates with indications for bariatric surgery, sleeve gastrectomy is the preferred approach as it preserves endoscopic access to the biliary tree, avoids malabsorption-related issues for immunosuppression medications, and poses less operative risk as compared with RYGB. Data to inform optimal timing of bariatric surgery relative to liver transplantation are sparse; however, recent consensus statements provide guidance based on the established literature (124). Bariatric surgery before liver transplant is generally safe in patients with low MELD scores and compensated cirrhosis although surgery should ideally be performed at an experienced, high-volume center (125). Weight loss may mitigate some transplant-related surgical risk, increase transplant access (126), and minimize risk of post-transplant recurrent metabolic dysfunction–associated steatohepatitis. Bariatric surgery at the time of liver transplant may be considered in selected patients at experienced centers, inclusive of those with high MELD scores that would otherwise preclude safe bariatric surgery before transplantation. Retrospective single-center studies demonstrate significant postoperative weight loss and improvement in metabolic comorbidities; however, this is balanced against longer operative times, higher risk of ICU stay, and higher rates of early postoperative complications (127,128). Bariatric surgery after liver transplant avoids surgical risk related to cirrhosis; however, it does not modify the problem of morbid obesity during waitlisting or the perioperative transplant period. Additional disadvantages include increased technical difficulties related to adhesions, higher reoperation rate, and increased postoperative morbidity. The role of bariatric surgery in the context of cirrhosis and liver transplantation continues to evolve, especially in light of weight loss pharmacotherapies such as glucagon-like peptide-1 receptor agonists which may induce weight loss and metabolic risk reduction even in patients with cirrhosis (129).
CONCLUSION
Surgical decision-making in patients with cirrhosis requires a nuanced, multidisciplinary approach that accounts for the severity of liver disease, the presence of portal hypertension, nonhepatic comorbidities, and the nature and setting of the planned procedure. In this guideline, we have provided evidence-based recommendations and key concepts to support individualized preoperative risk assessment and perioperative optimization. Use of validated cirrhosis-surgical risk scores; attention to modifiable risk factors such as frailty, nutrition, and hemostatic derangements; and consideration of procedural alternatives or referral to high-volume centers are central to improving outcomes. Through thoughtful integration of these principles into clinical practice, providers can enhance surgical safety and inform shared decision-making for this growing and vulnerable patient population.
CONFLICTS OF INTEREST
Guarantor of the article: Nadim Mahmud, MD, MS, MPH, MSCE.
Specific author contributions: All authors: conceptualization, manuscript drafting, editing, and final review.
Financial support: N. Mahmud reported receiving research support from the National Institute of Diabetes and Digestive and Kidney Diseases (K08-DK124577, R25-DK108711, R03-DK134794). He also reported investigator-initiated research funding from Grifols, unrelated to the current work. Z.P. Fricker disclosed institutional grants from Lipocine, Inc., River2Renal Corp, and Bausch Inc., as well as consulting fees from Guidepoint, Pick Research, and Atheneum. L.M. McElroy reported salary support from the National Institute on Minority Health and Health Disparities (K08-MD017632), the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK137110), and investigator-initiated research support from Veloxis. She also serves as Vice Chair of the OPTN Data Advisory Committee. R.J. Wong disclosed institutional research funding from Gilead Sciences, Exact Sciences, Theratechnologies, and Durect Corporation. He has also served as a consultant, without compensation, for Gilead Sciences, Salix Pharmaceuticals, and Mallinckrodt Pharmaceuticals.
Potential competing interests: None to report.